

Clinical Trials Supported

Community Partner
New Cancer Patients in 2024
Facility Info
- +34 91 550 48 00 ext: 2805
- Monday - Friday | 8am - 6pm CEST
- Av. de los Reyes Católicos, 2, 28040 Madrid, Spain
Founded in 2014, the START Madrid unit at Fundación Jiménez Díaz Quiron Salud Hospital was the first START research facility at a public teaching hospital in Europe. Lead by Dr. Victor Moreno, the FJD site has grown considerably, and now includes five principal investigators, over 60 dedciated clinical research staff, a drug development fellowship program, and a hematologic malignancies research team.
Explore START Madrid - FJD's Clinical Trials
Phase I Clinical Trials
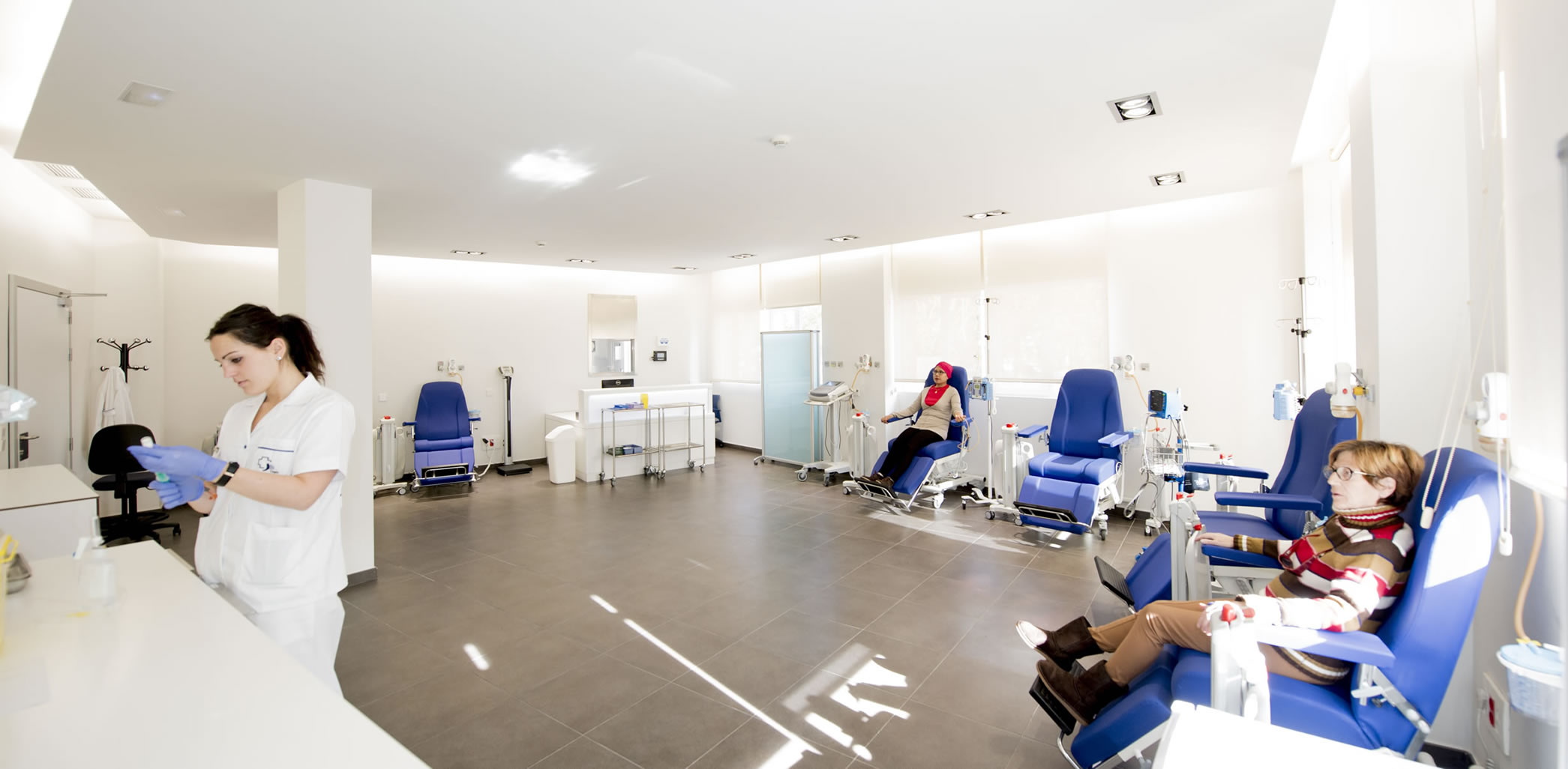
The START Madrid – FJD clinic has a dedicated treatment room that includes exam rooms, treatment chairs, infusion pumps, and local laboratory services.
Clinical Areas
- 4 exam rooms
- 8 treatment chairs
Clinical Laboratory
START has an on-site laboratory that can perform same day testing. This includes:
- CBC CMP
- PT/INR;aPTT
- Tumor markers
- Ua with microscopy
- Thyroid panels
- Select Hepatitis panels
- Troponin I
Investigational Drug Section (IDS)
Pharmacy Equipment
- Biological safety cabinets
- Negative pressure clean room
- Positive pressure anteroom
- Secure medication storage room with continuous temperature monitoring
- Pharmaceutical grade -20°C freezers
- Room temperature storage shelving (20-25°C)
- All equipment is annually inspected and calibrated
Pharmacokinetics
Biopsy Specimens
PK department staff are IATA shipping certified. Specimens may be shipped same day, weekly, or monthly.
Laboratory Equipment
All equipment is annually inspected and calibrated and includes the following:
- Refrigerated / room temperature centrifuges
- Microfuges with refrigeration/room temp.
- -80°C / -20°C freezer with continuous temperature monitoring
- Refrigerator
- Room temperature storage shelving
- Dry ice delivered twice a week
Regulatory Affairs & Data Management
The Regulatory Affairs Department ensures compliance with all regulatory and industry requirements by following FDA and GCP guidelines for every study.
- Rapid IRB review and approval turnaround
- Competitive start-up timelines
The dedicated Data Management team ensures clean, accurate, and timely data entry and query resolution.
- Experience with a wide variety of electronic data capture systems (i.e., Medidata RAVE, InForm, Veeva, etc.)
- Monitoring suites with high speed Internet access
facility Tour of START Madrid - FJD
facility Tour of START Madrid - FJD








