XenoSTART
Accelerating Translational Research for Breakthrough Oncology Treatments
Discover how our preclinical business, XenoSTART, can elevate your drug development programs with our extensive collection of over 2,500 high-quality PDX models.
Superior Patient-Derived Models: Powering Translational Research Excellence
At XenoSTART, our unique differentiation lies in our superior patient-derived xenograft (PDX) models. With the largest collection in the industry meticulously sourced from our global network of community-based oncology centers, our models serve as the foundation of our research.
Model Portfolio Highlights:
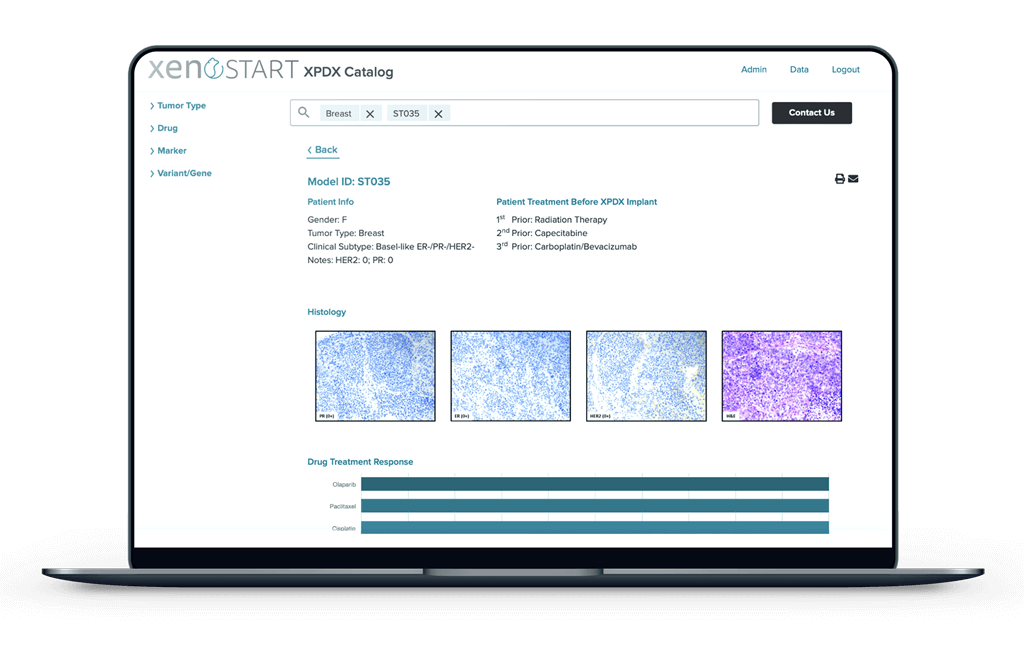
Explore our Catalog of Models
The Broadest Set of Indication Models in the Industry
- Adenoid Cystic Carcinoma
- Ampullary
- Bile Duct
- Bladder
- Bowel
- Brain
- Breast
- Cervix
- Chordoma
- Colorectal
- Dermatologic
- Endocrine
- Esophageal
- Gastric
- Head/Neck
- Hematologic
- Liver
- Lung
- Ovary
- Pancreas
- Penile
- Peritoneal
- Prostate
- Renal
- Sarcoma
- Uterine
- Vulva
Why Partner with XenoSTART?
01.
Unparalleled Expertise
Our team of world-class clinical investigators and research staff brings deep scientific expertise to every project, delivering high-quality results and insights.
02.
Extensive Patient-Derived Models
Boasting the largest collection of PDX models in the industry, sourced from diverse oncology centers worldwide, we provide comprehensive data across all tumor types.
03.
Cutting-Edge Facilities
Our state-of-the-art laboratories and advanced equipment support rigorous clinical pathology, treatment history analysis, and molecular characterization, ensuring the highest quality data.
04.
Streamlined Project Management
We prioritize efficient project management, clear communication, and flexibility to meet your specific needs and goals, ensuring seamless collaboration and timely results delivery.
Partnering with XenoSTART has been a game-changer for our drug development programs. Their expertise, extensive collection of patient-derived models, and commitment to operational excellence have accelerated our research and provided us with valuable insights. XenoSTART’s collaborative approach and dedication to quality make them an ideal service provider for any sponsor looking to advance their oncology research.
– Global Pharmaceutical Company
Head of Translational Research
Frequently Asked Questions
The Basics of PDX Models
What is a PDX model, and why is it used in preclinical studies?
Patient-derived xenograft (PDX) models are generated by directly transplanting human cancer samples into an immunodeficient host organism. The resulting models preserve the original human tumors’ cellular architecture and molecular heterogeneity. As a result, these preclinical models mimic the clinical situation of the patients from which they are derived.
What’s the difference between PDX models and other xenograft model types?
Compared to PDX models, other xenograft models – such as cell line-derived xenograft (CDX) models – are less heterogeneous and complex at the cellular and molecular level. Therefore, PDX models are considered more predictive of the clinical outcome for preclinical drug candidates than CDX models.
Why are PDX models important in cancer research?
Because PDX models closely mimic human cancer biology, they are more accurate predictors of preclinical responses to drug candidates. PDX models also aid in identifying drug-resistance mechanisms, tumor metastasis, and the development of personalized cancer treatments.
What are the advantages of using PDX models and tumor organoids?
Tumor organoids are patient-derived three-dimensional cell cultures that serve as simplified versions of tumors. They can be grown and manipulated in vitro for high-throughput drug screening and mimic a tumor’s functional, biological, and structural complexity. When combined with PDX models, tumor organoids can provide accurate and personalized insight into the potential efficacy of preclinical drug candidates.
How long does it take to develop a PDX model?
In our and others’ experience, it typically takes 2-8 months to engraft and grow a PDX model. For several tumor types and subtypes, the engraftment failure rate is high and may take longer than usual to develop into a reliable preclinical PDX model.
The XenoSource PDX Catalog
What types of PDX models are in your catalog?
There are over 2,500 PDX models across more than 25 tumor types annotated in XenoSource, our online PDX catalog. On average, we add 5-10 new PDX models each month to our collection.
Can my team browse the various models you have in your PDX collection?
Absolutely! Just request access to XenoSource using your work email address, and one of our team members will send you personalized email access. Within the catalog, you’ll see all of our models and associated metadata, including tumor type, clinical treatment history, DNA/RNA sequencing, and more.
How do I select the best PDX model from XenoSource for my study?
Once you receive access to XenoSource, you can browse the different models by selecting different tumor types, drug pretreatments, genetic markers or variants, and/or gene expression. Choose a model (or models) that closely match the clinical characteristics and genetic profile of your target indications. Within XenoSource, you can view model growth, histology, and DNA/RNA sequencing data. Our scientific team can also help you navigate model selection, providing industry expertise and information on model availability, timing, and budget.
I don’t see the model I want in XenoSource. Do you do custom model development?
We specialize in generating models that are notoriously difficult to grow and have a collection of uncommon and complex models that you likely won’t find anywhere else. If you have a model in mind but don’t see it in our database, contact us to see if we can initiate a custom model development project.
Working With XenoSTART
How do I start a PDX study with XenoSTART?
We offer flexible engagement models to fit the needs of your preclinical drug development program, including outsourcing your studies to us, licensing PDX models for internal use, and custom model development. If outsourcing to XenoSTART, we perform a detailed protocol review with you and our scientific team to ensure we clearly understand the objectives and design. We then develop a statement of work, including specific services, timelines, and deliverables. If you are licensing our models, we can provide MTAs, technical guidance, and more.
What types of preclinical studies can you perform at XenoSTART?
Our scientific team can test the efficacy and/or PK/PD of preclinical and clinical drug candidates and perform PDX efficacy screens focused on target, patient pretreatment, or indication.
What PDX implantation method do you use?
We implant all of the PDX models within our catalog using subcutaneous engraftment.
How do you monitor tumor growth during PDX studies?
We monitor tumor growth with manual calipers to measure the length and width of implanted tumors over time. This raw data is used to calculate tumor volume growth curves. Study results are represented by calculating tumor growth inhibition (TGI), which means the % change in tumor volumes in treatment arms relative to control.
Do you design and perform studies that test the efficacy of immunotherapies using humanized models?
We don’t create humanized models in-house. We do, however, have a trusted partner network with expertise in generating humanized mouse models and rely on them to develop humanized PDX models and perform preclinical studies to test the efficacy of immunotherapies. As with our non-humanized PDX studies, these are managed by our team of scientific experts, providing high-quality, preclinical data and services.
What’s the average timeline for a preclinical study, from kick-off to data delivery?
Following the full execution of an SOW, the average timeline for Day 0 dosing of a preclinical study is 60 days.
What kind of characterization data can you provide for your PDX models?
For all of the PDX models in our catalog, we provide detailed tumor growth data, donor patient medical/treatment history, DNA/RNA sequencing data, histology, and more.
How is study data reported throughout the study?
Once a study is initiated, you’ll have access to a data dashboard to access your study in real time. We also plan bimonthly meetings to review your data and answer your team’s questions. At the end of a study, we deliver the raw data to you but can also create a tailored study report by request. We have medical writing capabilities to support the preparation of preclinical data for IND submission.
XenoSTART and START
What’s the relationship between XenoSTART and The START Center for Cancer Research (“START”)?
START is the clinical division of XenoSTART. It’s the largest early-phase oncology trial site organization globally and a key partner to pharma and biotech sponsors. Positioned at the leading edge of clinical drug development, START and our PIs provide unique clinical insights into emerging patient and treatment trends to XenoSTART. These insights enable XenoSTART to pursue more clinically relevant tissue sourcing, preclinical PDX model development, and optimized study design. Currently, START operates 8 clinical locations across the U.S. and Europe but is continuously expanding, creating broader access to diverse patient samples and PDX models.
XenoSTART News & Blog
Blog
Sourcing Better PDX Models with a Streamlined “Clinic-to-Bench” Pipeline
Discover how XenoSTART’s clinic-to-bench pipeline delivers more clinically relevant PDX models, real-world patient data, and accelerated drug development. Patient-derived xenograft …
Blog
Capturing the Complexity of Real Cancer Cases with Patient-Derived Xenograft Models
Capturing the Complexity of Real Cancer Cases with Patient-Derived Xenograft Models One of the most significant areas of advancement in …
Webinar
Overcoming Resistance: Enhancing CDK4/6 Inhibitor Efficacy in Advanced Breast Cancer Treatment
Blog
Sourcing Better PDX Models with a Streamlined “Clinic-to-Bench” Pipeline
Discover how XenoSTART’s clinic-to-bench pipeline delivers more clinically relevant PDX models, real-world patient data, and accelerated drug development. Patient-derived xenograft …
Blog
Capturing the Complexity of Real Cancer Cases with Patient-Derived Xenograft Models
Capturing the Complexity of Real Cancer Cases with Patient-Derived Xenograft Models One of the most significant areas of advancement in …
Webinar
Overcoming Resistance: Enhancing CDK4/6 Inhibitor Efficacy in Advanced Breast Cancer Treatment
Let's Talk
Ready to Partner with XenoSTART?
Contact XenoSTART today to discuss how our preclinical research services can support your oncology drug development programs and bring innovative treatments to patients.
